CE Marking for Face Masks
There are two types of EU legal Frameworks applicable are:
- Regulation (EU) 2016/425: For face mask as PPE
- Regulation (EU) 2017/745 (superseded from Council directive 93/42/EEC): For medical gloves, surgical masks, intensive care & other medical equipment that come under the scope of EU legal framework on medical devices.
| Face Mask Type | Regulation | Standard | NB Involvement | Testing Required |
| Surgical masks | Regulation (EU) 2016/425 | EN 14683 | Not Needed | By Independent Lab |
| Face masks | Regulation (EU) 2017/745 | EN 149+ EN 14683 | In Design Examination | By NB Laboratory |
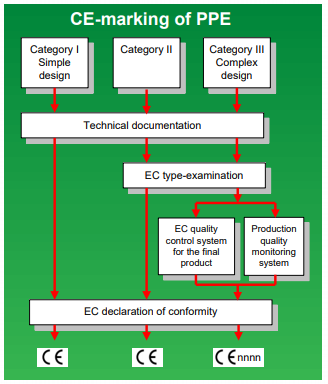
Applicable EU Standards
- Under PPE, any technical solution may be used by manufacturer to meet the essential requirement.
- For Face Masks,
- EN 149:2001+A1:2009 for FFP Type mask
- EN 14683:2019 for Surgical Type mask

